Modern evolutionary synthesis
| Part of the Biology series on |
| Evolution |
| Mechanisms and processes |
|---|
|
Adaptation |
| Research and history |
|
Introduction |
| Evolutionary biology fields |
|
Cladistics |
| Biology portal · |
The modern evolutionary synthesis is also referred to as the new synthesis, the modern synthesis, the evolutionary synthesis and the neo-darwinian synthesis. It is a union of ideas from several biological specialties which provides a widely accepted account of evolution. The synthesis has been accepted by nearly all working biologists.[1] The synthesis was produced over a decade (1936–1947). The previous development of population genetics (1918–1932) was a stimulus, as it showed that Mendelian genetics was consistent with natural selection and gradual evolution. The synthesis is still, to a large extent, the current paradigm in evolutionary biology.[2]
Julian Huxley invented the term, when he produced his book, Evolution: The Modern Synthesis (1942). Other major figures in the modern synthesis include R. A. Fisher, Theodosius Dobzhansky, J.B.S. Haldane, Sewall Wright, E.B. Ford, Ernst Mayr, Bernhard Rensch, Sergei Chetverikov, George Gaylord Simpson, and G. Ledyard Stebbins.
The modern synthesis solved difficulties and confusions caused by the specialisation and poor communication between biologists in the early years of the 20th century. Discoveries of early geneticists were difficult to reconcile with gradual evolution and the mechanism of natural selection. The synthesis reconciled the two schools of thought, while providing evidence that studies of populations in the field were crucial to evolutionary theory. It drew together ideas from several branches of biology that had become separated, particularly genetics, cytology, systematics, botany, morphology, ecology and paleontology.
Contents |
Summary of the modern synthesis
The modern synthesis bridged the gap between experimental geneticists and naturalists, and between both and palaeontologists. It states that:[3][4][5]
- All evolutionary phenomena can be explained in a way consistent with known genetic mechanisms and the observational evidence of naturalists.
- Evolution is gradual: small genetic changes, recombination ordered by natural selection. Discontinuities amongst species (or other taxa) are explained as originating gradually through geographical separation and extinction (not saltation).
- Natural selection is by far the main mechanism of change; even slight advantages are important when continued. The object of selection is the phenotype in its surrounding environment.
- The role of genetic drift is equivocal. Though strongly supported initially by Dobzhansky, it was downgraded later as results from ecological genetics were obtained.
- Thinking in terms of populations, rather than individuals, is primary: the genetic diversity existing in natural populations is a key factor in evolution. The strength of natural selection in the wild is greater than previously expected; the effect of ecological factors such as niche occupation and the significance of barriers to gene flow are all important.
- In palaeontology, the ability to explain historical observations by extrapolation from microevolution to macroevolution is proposed. Historical contingency means explanations at different levels may exist. Gradualism does not mean constant rate of change.
The idea that speciation occurs after populations are reproductively isolated has been much debated. In plants, polyploidy must be included in any view of speciation. Formulations such as 'evolution consists primarily of changes in the frequencies of alleles between one generation and another' were proposed rather later. The traditional view is that developmental biology ('evo-devo') played little part in the synthesis,[6] but an account of Gavin de Beer's work by Stephen J. Gould suggests he may be an exception.[7]
Developments leading up to the synthesis
1859–1899
Charles Darwin's The Origin of Species was successful in convincing most biologists that evolution had occurred, but was less successful in convincing them that natural selection was its primary mechanism. In the 19th and early 20th centuries, variations of Lamarckism, orthogenesis ('progressive' evolution), and saltationism (evolution by jumps) were discussed as alternatives.[8] Also, Darwin did not offer a precise explanation of how new species arise. As part of the disagreement about whether natural selection alone was sufficient to explain speciation, George Romanes coined the term neo-Darwinism to refer to the version of evolution advocated by Alfred Russel Wallace and August Weismann with its heavy dependence on natural selection.[9] Weismann and Wallace rejected the Lamarckian idea of inheritance of acquired characteristics, something that Darwin had not ruled out.[10]
Weismann's idea was that the relationship between the hereditary material, which he called the germ plasm (de: Keimplasma), and the rest of the body (the soma) was a one-way relationship: the germ-plasm formed the body, but the body did not influence the germ-plasm, except indirectly in its participation in a population subject to natural selection. Weismann was translated into English, and though he was influential, it took many years for the full significance of his work to be appreciated.[11] Later, after the completion of the modern synthesis, the term neo-Darwinism came to be associated with its core concept: evolution, driven by natural selection acting on variation produced by genetic mutation, and genetic recombination (chromosomal crossovers).[9]
1900–1915
Gregor Mendel's work was re-discovered by Hugo de Vries and Carl Correns in 1900. News of this reached William Bateson in England, who reported on the paper during a presentation to the Royal Horticultural Society in May 1900.[12] It showed that the contributions of each parent retained their integrity rather than blending with the contribution of the other parent. This reinforced a division of thought, which was already present in the 1890s.[13] The two schools were:
- Saltationism (large mutations or jumps), favored by early Mendelians who viewed hard inheritance as incompatible with natural selection[14]
- Biometric school: led by Karl Pearson and Walter Weldon, argued vigorously against it, saying that empirical evidence indicated that variation was continuous in most organisms, not discrete as Mendelism predicted.
The relevance of Mendelism to evolution was unclear and hotly debated, especially by Bateson, who opposed the biometric ideas of his former teacher Weldon. Many scientists believed the two theories substantially contradicted each other.[15] This debate between the biometricians and the Mendelians continued for some 20 years and was only solved by the development of population genetics.
T. H. Morgan began his career in genetics as a saltationist, and started out trying to demonstrate that mutations could produce new species in fruit flies. However, the experimental work at his lab with Drosophila melanogaster, which helped establish the link between Mendelian genetics and the chromosomal theory of inheritance, demonstrated that rather than creating new species in a single step, mutations increased the genetic variation in the population.[16]
The foundation of population genetics
The first step towards the synthesis was the development of population genetics. R.A. Fisher, J.B.S. Haldane, and Sewall Wright provided critical contributions. In 1918, Fisher produced the paper "The Correlation Between Relatives on the Supposition of Mendelian Inheritance",[17] which showed how the continuous variation measured by the biometricians could be the result of the action of many discrete genetic loci. In this and subsequent papers culminating in his 1930 book The Genetical Theory of Natural Selection, Fisher was able to show how Mendelian genetics was, contrary to the thinking of many early geneticists, completely consistent with the idea of evolution driven by natural selection.[18] During the 1920s, a series of papers by J.B.S. Haldane applied mathematical analysis to real world examples of natural selection such as the evolution of industrial melanism in peppered moths.[18] Haldane established that natural selection could work in the real world at a faster rate than even Fisher had assumed.[19]
Sewall Wright focused on combinations of genes that interacted as complexes, and the effects of inbreeding on small relatively isolated populations, which could exhibit genetic drift. In a 1932 paper he introduced the concept of an adaptive landscape in which phenomena such as cross breeding and genetic drift in small populations could push them away from adaptive peaks, which would in turn allow natural selection to push them towards new adaptive peaks.[18] Wright's model would appeal to field naturalists such as Theodosius Dobzhansky and Ernst Mayr who were becoming aware of the importance of geographical isolation in real world populations.[19] The work of Fisher, Haldane and Wright founded the discipline of population genetics. This is the precursor of the modern synthesis, which is an even broader coalition of ideas.[18][19][20]
The modern synthesis
Theodosius Dobzhansky, an Ukrainian emigrant, who had been a postdoctoral worker in Morgan's fruit fly lab, was one of the first to apply genetics to natural populations. He worked mostly with Drosophila pseudoobscura. He says pointedly: "Russia has a variety of climates from the Arctic to sub-tropical... Exclusively laboratory workers who neither possess nor wish to have any knowledge of living beings in nature were and are in a minority".[21] Not surprisingly, there were other Russian geneticists with similar ideas, though for some time their work was known to only a few in the West. His 1937 work Genetics and the Origin of Species was a key step in bridging the gap between population geneticists and field naturalists. It presented the conclusions reached by Fisher, Haldane, and especially Wright in their highly mathematical papers in a form that was easily accessible to others. It also emphasized that real world populations had far more genetic variability than the early population geneticists had assumed in their models, and that genetically distinct sub-populations were important. Dobzhansky argued that natural selection worked to maintain genetic diversity as well as driving change. Dobzhansky had been influenced by his exposure in the 1920s to the work of a Russian geneticist named Sergei Chetverikov who had looked at the role of recessive genes in maintaining a reservoir of genetic variability in a population before his work was shut down by the rise of Lysenkoism in the Soviet Union.[18][19]
Edmund Brisco Ford's work complemented that of Dobzhansky. It was as a result of Ford's work, as well as his own, that Dobzhansky changed the emphasis in the third edition of his famous text from drift to selection.[22] Ford was an experimental naturalist who wanted to test natural selection in nature. He virtually invented the field of research known as ecological genetics. His work on natural selection in wild populations of butterflies and moths was the first to show that predictions made by R.A. Fisher were correct. He was the first to describe and define genetic polymorphism, and to predict that human blood group polymorphisms might be maintained in the population by providing some protection against disease.[23]
Ernst Mayr's key contribution to the synthesis was Systematics and the Origin of Species, published in 1942. Mayr emphasized the importance of allopatric speciation, where geographically isolated sub-populations diverge so far that reproductive isolation occurs. He was sceptical of the reality of sympatric speciation believing that geographical isolation was a prerequisite for building up intrinsic (reproductive) isolating mechanisms. Mayr also introduced the biological species concept that defined a species as a group of interbreeding or potentially interbreeding populations that were reproductively isolated from all other populations.[18][19][24] Before he left Germany for the United States in 1930, Mayr had been influenced by the work of German biologist Bernhard Rensch. In the 1920s Rensch, who like Mayr did field work in Indonesia, analyzed the geographic distribution of polytypic species and complexes of closely related species paying particular attention to how variations between different populations correlated with local environmental factors such as differences in climate. In 1947, Rensch published Neuere Probleme der Abstammungslehre: die Transspezifische Evolution (English translation 1959: Evolution above the Species level). This looked at how the same evolutionary mechanisms involved in speciation might be extended to explain the origins of the differences between the higher level taxa. His writings contributed to the rapid acceptance of the synthesis in Germany.[25][26]
George Gaylord Simpson was responsible for showing that the modern synthesis was compatible with paleontology in his book Tempo and Mode in Evolution published in 1944. Simpson's work was crucial because so many paleontologists had disagreed, in some cases vigorously, with the idea that natural selection was the main mechanism of evolution. It showed that the trends of linear progression (in for example the evolution of the horse) that earlier paleontologists had used as support for neo-Lamarckism and orthogenesis did not hold up under careful examination. Instead the fossil record was consistent with the irregular, branching, and non-directional pattern predicted by the modern synthesis.[18][19]
The botanist G. Ledyard Stebbins was another major contributor to the synthesis. His major work, Variation and Evolution in Plants, was published in 1950. It extended the synthesis to encompass botany including the important effects of hybridization and polyploidy in some kinds of plants.[18]
Further advances
The modern evolutionary synthesis continued to be developed and refined after the initial establishment in the 1930s and 1940s. The work of W. D. Hamilton, George C. Williams, John Maynard Smith and others led to the development of a gene-centric view of evolution in the 1960s. The synthesis as it exists now has extended the scope of the Darwinian idea of natural selection to include subsequent scientific discoveries and concepts unknown to Darwin, such as DNA and genetics, which allow rigorous, in many cases mathematical, analyses of phenomena such as kin selection, altruism, and speciation.
A particular interpretation most commonly associated with Richard Dawkins, author of The Selfish Gene, asserts that the gene is the only true unit of selection.[27] Dawkins further extended the Darwinian idea to include non-biological systems exhibiting the same type of selective behavior of the 'fittest' such as memes in culture. The synthesis continues to undergo regular review.[28] (See also Current research in evolutionary biology).
After the synthesis
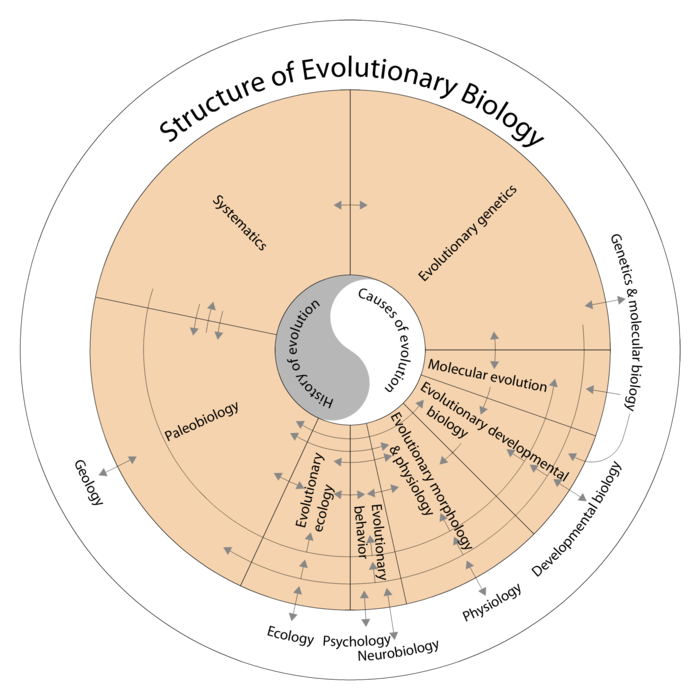
The history and causes of evolution (center) are subject to various subdisciplines of evolutionary biology. The areas of segments give an impression of the contributions of subdisciplines to the literature of evolutionary biology.
There are a number of discoveries in earth sciences and biology which have arisen since the synthesis. Listed here are some of those topics which are relevant to the evolutionary synthesis, and which seem soundly based.
Understanding of Earth history
The Earth is the stage on which the evolutionary play is performed. Darwin studied evolution in the context of Charles Lyell's geology, but our present understanding of Earth history includes some critical advances made during the last half-century.
- The age of the Earth has been revised upwards. It is now estimated at 4.56 billion years, about one-third of the age of the universe. It is worth noting that the Phanerozoic only occupies the last 1/9th of this period of time.[29]
- The triumph of Alfred Wegener's idea of continental drift came around 1960. The key principle of plate tectonics is that the lithosphere exists as separate and distinct tectonic plates, which ride on the fluid-like (visco-elastic solid) asthenosphere. This discovery provides a unifying theory for geology, linking phenomena such as volcanos, earthquakes, orogeny, and providing data for many paleogeographical questions.[30] One major question is still unclear: when did plate tectonics begin?[31]
- Our understanding of the evolution of the Earth's atmosphere has progressed. The substitution of oxygen for carbon dioxide in the atmosphere, which occurred in the Proterozoic, caused probably by cyanobacteria in the form of stromatolites, caused changes leading to the evolution of aerobic organisms.[32][33]
- The identification of the first generally accepted fossils of microbial life was made by geologists. These rocks have been dated as about 3.465 billion years ago.[34] Walcott was the first geologist to identify pre-Cambrian fossil bacteria from microscopic examination of thin rock slices. He also thought stromatolites were organic in origin. His ideas were not accepted at the time, but may now be appreciated as great discoveries.[35]
- Information about paleoclimates is increasingly available, and being used in paleontology. One example: the discovery of massive ice ages in the Proterozoic, following the great reduction of CO2 in the atmosphere. These ice ages were immensely long, and led to a crash in microflora.[36] See also Cryogenian period and Snowball Earth.
- Catastrophism and mass extinctions. A partial reintegration of catastrophism has occurred,[37] and the importance of mass extinctions in large-scale evolution is now apparent. Extinction events disturb relationships between many forms of life and may remove dominant forms and release a flow of adaptive radiation amongst groups that remain. Causes include meteorite strikes (K–T junction; Upper Devonian); flood basalt provinces (Deccan traps at K/T junction; Siberian traps at P–T junction); and other less dramatic processes.[38][39]
Conclusion: Our present knowledge of earth history strongly suggests that large-scale geophysical events influenced macroevolution and megaevolution. These terms refer to evolution above the species level, including such events as mass extinctions, adaptive radiation, and the major transitions in evolution.[40][41]
Symbiotic origin of eukaryotic cell structures
Once symbiosis was discovered in lichen and in plant roots (rhizobia in root nodules) in the 19th century, the idea arose that the process might have occurred more widely, and might be important in evolution. Anton de Bary invented the concept of symbiosis;[42] several Russian biologists promoted the idea;[43] Edwin Wilson mentioned it in his epic text The Cell;[44] a book was published in the U.S.A. in 1927 with the title Symbionticism and the origin of species;[45] and there was a brief mention by Julian Huxley in 1930;[46] all in vain because sufficient evidence was lacking. Symbiosis as a major evolutionary force was not discussed at all in the evolutionary synthesis.[47]
The role of symbiosis in cell evolution was revived partly by Joshua Lederberg,[48] and finally brought to light by Lynn Margulis in a series of papers and books.[49][50] It turns out that some cell organelles are of microbial origin: mitochondria and chloroplasts definitely, cilia, flagella and centrioles possibly, and perhaps the nuclear membrane and much of the chromosome structure as well. What is now clear is that the evolution of eukaryote cells is either caused by, or at least profoundly influenced by, symbiosis with bacterial and archaean cells in the Proterozoic.
The origin of the eukaryote cell by symbiosis in several stages was not part of the evolutionary synthesis. It is, at least on first sight, an example of megaevolution by big jumps. However, what symbiosis provided was a copious supply of heritable variation from microorganisms, which was fine-tuned over a long period to produce the cell structure we see today. This part of the process is consistent with evolution by natural selection.[51]
Trees of life
The ability to analyse sequence in macromolecules (protein, DNA, RNA) provides evidence of descent, and permits us to work out genealogical trees covering the whole of life, since now there are data on every major group of living organisms. This project, begun in a tentative way in the 1960s, has become a search for the universal tree or the universal ancestor, a phrase of Carl Woese.[52][53] The tree that results has some unusual features, especially in its roots. There are two kingdoms of prokaryotes: bacteria and archaea, both of which contributed genetic material to the eukaryotes, mainly by means of symbiosis. Also, since bacteria can pass genetic material to other bacteria, their relationships look more like a web than a tree. Once eukaryotes were established, their sexual reproduction produced the traditional branching tree-like pattern, the only diagram Darwin put in the Origin. The last universal ancestor (LUA) would be a prokaryotic cell before the split between the bacteria and archaea. LUA is defined as most recent organism from which all organisms now living on Earth descend (some 3.5 to 3.8 billion years ago, in the Archean era).[54][55]
This technique may be used to clarify relationships within any group of related organisms. It is now a standard procedure, and examples are published regularly. April 2009 sees the publication of a tree covering all the animal phyla, derived from sequences from 150 genes in 77 taxa.[56]
Early attempts to identify relationships between major groups were made in the 19th century by Ernst Haeckel, and by comparative anatomists such as Thomas Henry Huxley and E. Ray Lankester. Enthusiasm waned: it was often difficult to find evidence to adjudicate between different opinions. Perhaps for that reason, the evolutionary synthesis paid surprisingly little attention to this activity. It is certainly a lively field of research today.
Evo-devo
What once was called embryology played a modest role in the evolutionary synthesis,[57] mostly about evolution by changes in developmental timing (allometry and heterochrony).[58] Man himself was, according to Bolk, a typical case of evolution by retention of juvenile characteristics (neoteny). He listed many characters where "Man, in his bodily development, is a primate foetus that has become sexually mature".[59] Unfortunately, his interpretation of these ideas was non-Darwinian, but his list of characters is both interesting and convincing.[60]
Modern interest in Evo-devo springs from clear proof that development is closely controlled by special genetic systems, and the hope that comparison of these systems will tell us much about the evolutionary history of different groups.[61][62] In a series of experiments with the fruit-fly Drosophila, Edward B. Lewis was able to identify a complex of genes whose proteins bind to the cis-regulatory regions of target genes. The latter then activate or repress systems of cellular processes that accomplish the final development of the organism.[63][64] Furthermore, the sequence of these control genes show co-linearity: the order of the loci in the chromosome parallels the order in which the loci are expressed along the anterior-posterior axis of the body. Not only that, but this cluster of master control genes programs the development of all higher organisms.[65][66] Each of the genes contains a homeobox, a remarkably conserved DNA sequence. This suggests the complex itself arose by gene duplication.[67][68][69] In his Nobel lecture, Lewis said "Ultimately, comparisons of the [control complexes] throughout the animal kingdom should provide a picture of how the organisms, as well as the [control genes] have evolved".
The term deep homology was coined to describe the common origin of genetic regulatory apparatus used to build morphologically and phylogenetically disparate animal features.[70] It applies when a complex genetic regulatory system is inherited from a common ancestor, as it is in the evolution of vertebrate and invertebrate eyes. The phenomenon is implicated in many cases of parallel evolution.[71]
A great deal of evolution may take place by changes in the control of development. This may be relevant to punctuated equilibrium theory, for in development a few changes to the control system could make a significant difference to the adult organism. An example is the giant panda, whose place in the mammalia was long uncertain.[72] Apparently, the giant panda's evolution from Ursus to Ailuropoda required the change of only a few genetic messages (5 or 6 perhaps), yet the phenotypic and lifestyle change from a standard bear is considerable.[73][74] The transition could therefore be effected relatively swiftly.
Fossil discoveries
In the past thirty or so years there have been excavations in parts of the world which had scarcely been investigated before. Also, there is fresh appreciation of fossils discovered in the 19th century, but then denied or deprecated: the classic example is the Ediacaran biota from the immediate pre-Cambrian, after the Cryogenian period. These soft-bodied fossils are the first record of multicellular life. The interpretation of this fauna is still in flux.
Many outstanding discoveries have been made, and some of these have implications for evolutionary theory. The discovery of feathered dinosaurs and early birds from the Lower Cretaceous of Liaoning, N.E. China have convinced most students that birds did evolve from coelurosaurian theropod dinosaurs. Less well known, but perhaps of equal evolutionary significance, are the studies on early insect flight, on stem tetrapods from the Upper Devonian,[75][76] and the early stages of whale evolution.[77]
A shaft of light has been thrown on the evolution of flatfish (pleuronectiformes), such as plaice, sole, turbot and halibut, by recent work. Flatfish are interesting because they are one of the few vertebrate groups with external asymmetry. Their young are perfectly symmetrical, but the head is remodelled during a metamorphosis, which entails the migration of one eye to the other side, close to the other eye. Some species have both eyes on the left (turbot), some on the right (halibut, sole); all living and fossil flatfish to date show an 'eyed' side and a 'blind' side.[78] The lack of an intermediate condition in living and fossil flatfish species had led to debate about the origin of such a striking adaptation. The case was considered by Lamark,[79] who thought flatfish precursors would have lived in shallow water for a long period, and by Darwin, who predicted a gradual migration of the eye, mirroring the metamorphosis of the living forms. Darwin's long-time critic St. George Mivart thought that the intermediate stages could have no selective value,[80] and in the 6th edition of the Origin, Darwin made a concession to the possibility of acquired traits.[81] Many years later the geneticist Richard Goldschmidt put the case forward as an example of evolution by saltation, bypassing intermediate forms.[82][83]
A recent examination of two fossil species from the Eocene has provided the first clear picture of flatfish evolution. The discovery of stem flatfish with incomplete orbital migration refutes Goldschmidt's ideas, and demonstrates that "the assembly of the flatfish bodyplan occurred in a gradual, stepwise fashion".[84] There are no grounds for thinking that incomplete orbital migration was maladaptive, because stem forms with this condition ranged over two geological stages, and are found in localities which also yield flatfish with the full cranial asymmetry. The evolution of flatfish falls squarely within the evolutionary synthesis.[78]
See also
- Evolution
- The Origin of Species
- History of evolutionary thought
- Gene-centered view of evolution
- Particulate inheritance theory
- Population genetics
- Developmental systems theory
- Polymorphism (biology)
Footnotes
- ↑ "The scientific consensus around evolution is overwhelming". "Appendix: Frequently Asked Questions" (php). Science and Creationism: A View from the National Academy of Sciences (Second ed.). Washington, DC: The National Academy of Sciences. 1999. p. 28. ISBN ISBN-0-309-06406-6. http://www.nap.edu/openbook.php?record_id=6024&page=27#p200064869970027001. Retrieved September 24, 2009.
- ↑ Mayr, Ernst 2002. What evolution is. Weidenfeld & Nicolson, London. p270
- ↑ Huxley J.S. 1942. Evolution: the modern synthesis. Allen & Unwin, London. 2nd ed 1963; 3rd ed 1974.
- ↑ Mayr & Provine 1998
- ↑ Mayr E. 1982. The growth of biological thought: diversity, evolution & inheritance. Harvard, Cambs. p567 et seq.
- ↑ Smocovitis, V. Betty. 1996. Unifying Biology: the evolutionary synthesis and evolutionary biology. Princeton University Press. p192
- ↑ Gould S.J. Ontogeny and phylogeny. Harvard 1977. p221-2
- ↑ Bowler P.J. 2003. Evolution: the history of an idea. pp236–256
- ↑ 9.0 9.1 Gould The Structure of Evolutionary Theory p. 216
- ↑ Kutschera U. 2003. A comparative analysis of the Darwin-Wallace papers and the development of the concept of natural selection. Theory in Biosciences 122, 343-359
- ↑ Bowler pp. 253–256
- ↑ Mike Ambrose. "Mendel's Peas". Genetic Resources Unit, John Innes Centre, Norwich, UK. http://www.jic.ac.uk/germplas/pisum/zgs4f.htm. Retrieved 2007-09-22.
- ↑ Bateson, William 1894. Materials for the study of variation, treated with special regard to discontinuity in the origin of species. The division of thought was between gradualists of the Darwinian school, and saltationists such as Bateson. Mutations (as 'sports') and polymorphisms were well known long before the Mendelian recovery.
- ↑ Larson pp. 157–166
- ↑ Grafen, Alan; Ridley, Mark (2006). Richard Dawkins: How A Scientist Changed the Way We Think. New York, New York: Oxford University Press. p. 69. ISBN 0199291160.
- ↑ Bowler pp. 271–272
- ↑ Transactions of the Royal Society of Edinburgh, 52:399–433
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 18.7 Larson Evolution: The Remarkable History of a Scientific Theory pp. 221–243
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 Bowler Evolution: The history of an Idea pp. 325–339
- ↑ Gould The Structure of Evolutionary Theory pp. 503–518
- ↑ Mayr & Provine 1998 p. 231
- ↑ Dobzhansky T. 1951. Genetics and the Origin of Species. 3rd ed, Columbia University Press N.Y.
- ↑ Ford E.B. 1964, 4th edn 1975. Ecological genetics. Chapman and Hall, London.
- ↑ Mayr and Provine 1998 pp. 33–34
- ↑ Smith, Charles H.. "Rensch, Bernhard (Carl Emmanuel) (Germany 1900–1990)". Western Kentucky University. http://www.wku.edu/~smithch/chronob/RENS1900.htm. Retrieved 2007-09-22.
- ↑ Mayr and Provine 1998 pp. 298–299, 416
- ↑ Bowler p.361
- ↑ Pigliucci, Massimo 2007. Do we need an extended evolutionary synthesis? Evolution 61 12, 2743–2749.
- ↑ Dalrymple, G. Brent 2001. The age of the Earth in the twentieth century: a problem (mostly) solved. Special Publications, Geological Society of London 190, 205–221.
- ↑ Van Andel, Tjeerd 1994. New views on an old planet: a history of global change. 2nd ed. Cambridge.
- ↑ Witz A. 2006. The start of the world as we know it. Nature 442, p128.
- ↑ Schopf J.W. and Klein (eds) 1992. The Proterozoic biosphere: a multi-disciplinary study. Cambridge University Press.
- ↑ Lane, Nick 2002. Oxygen: the molecule that made the world. Oxford.
- ↑ Schopf J.W. 1999. Cradle of life: the discovery of Earth's earliest fossils. Princeton.
- ↑ Yochelson, Ellis L. 1998. Charles Doolittle Walcott: paleontologist. Kent State, Ohio.
- ↑ Knoll A.H. and Holland H.D. 1995. Oxygen and Proterozoic evolution: an update. In National Research Council, Effects of past climates upon life. National Academy, Washington D.C.
- ↑ Huggett, Richard J. 1997. Catastrophism. new ed. Verso.
- ↑ Hallam A. and Wignall P.B. 1997. Mass extinctions and their aftermath. Columbia, N.Y.
- ↑ Elewa A.M.T. (ed) 2008. Mass extinctions. Springer, Berlin.
- ↑ The terms (or their equivalents) were used as part of the synthesis by Simpson G.G. 1944. Tempo and mode in evolution, and Rensch B. 1947. Evolution above the species level. Columbia, N.Y. They were also used by some non-Darwinian evolutionists such as Yuri Filipchenko and Richard Goldschmidt. Here we use the terms as part of the evolutionary synthesis: they do not imply any change in mechanism.
- ↑ Maynard Smith J. and Szathmáry E. 1997. The major transitions in evolution. Oxford.
- ↑ de Bary, H.A. 1879. Die Erscheinung der Symbiose. Strassburg.
- ↑ Khakhina, Liya Nikolaevna 1992. Concepts of symbiogenesis: a historical and critical study of the research of Russian scientists.
- ↑ Wilson E.B. 1925. The cell in development and heredity . Macmillan, N.Y.
- ↑ Walin I.E. 1927. Symbionticism and the origin of species. Williams & Wilkins, Baltimore.
- ↑ Wells H.G., Huxley J. and Wells G.P. 1930. The science of life. London vol 2, p505. This section (The ABC of genetics) was written by Huxley.
- ↑ Sapp, January 1994. Evolution by association: a history of symbiosis. Oxford.
- ↑ Lederberg J. 1952. Cell genetics and hereditary symbiosis. Physiological Reviews 32, 403–430.
- ↑ Margulis L and Fester R (eds) 1991. Symbiosis as a source of evolutionary innovation. MIT.
- ↑ Margulis L. 1993. Symbiosis in cell evolution: microbial communities in the Archaean and Proterozoic eras. Freeman, N.Y.
- ↑ Maynard Smith J. and Szathmáry E. 1997. The major transitions in evolution. Oxford. The origin of the eukaryote cell is one of the seven major transitions, according to these authors.
- ↑ Woese, Carl 1998. The Universal Ancestor. PNAS 95, 6854–6859.
- ↑ Doolittle, W. Ford 1999. Phylogenetic classification and the Universal Tree. Science 284, 2124–2128.
- ↑ Doolittle, W. Ford 2000. Uprooting the tree of life. Scientific American 282 (6): 90–95.
- ↑ Villarreal LP, Witzany G. 2010. Viruses are essential agents within the roots and stem of the tree of life. Journal of Theoretical Biology 262(4): 698-710.
- ↑ Dunn, Casey W. et al 2009. Broad phylogenetic sampling improves resolution of the animal tree of life. Nature 452, 745–749.
- ↑ Laubichler M. and Maienschein J. 2007. From Embryology to Evo-Devo: a history of developmental evolution. MIT.
- ↑ de Beer, Gavin 1930. Embryology and evolution. Oxford; 2nd ed 1940 as Embryos and ancestors; 3rd ed 1958, same title.
- ↑ Bolk, L. 1926. Der Problem der Menschwerdung. Fischer, Jena.
- ↑ short-list of 25 characters reprinted in Gould, Stephen Jay 1977. Ontogeny and phylogeny. Harvard. p357
- ↑ Raff R.A. and Kaufman C. 1983. Embryos, genes and evolution: the developmental-genetic basis of evolutionary changes. Macmillan, N.Y.
- ↑ Carroll, Sean B. 2005. Endless forms most beautiful: the new science of Evo-Devo and the making of the animal kingdom. Norton, N.Y.
- ↑ Lewis E.B. 1995. The bithorax complex: the first fifty years. Nobel Prize lecture. Repr. in Ringertz N. (ed) 1997. Nobel lectures, Physiology or Medicine. World Scientific, Singapore.
- ↑ Lawrence P. 1992. The making of a fly. Blackwell, Oxford.
- ↑ Duncan I. 1987. The bithorax complex. Ann. Rev. Genetics 21, 285–319.
- ↑ Lewis E.B. 1992. Clusters of master control genes regulate the development of higher organisms. J. Am. Medical Assoc. 267, 1524–1531.
- ↑ McGinnis W. et al 1984. A conserved DNA sequence in homeotic genes of the Drosophila antennipedia and bithorax complexes. Nature 308, 428–433.
- ↑ Scott M.P. and Weiner A.J. 1984. Structural relationships among genes that control developmental sequence homology between the antennipedia, ultrabithorax and fushi tarazu loci of Drosophila. PNAS USA 81, 4115.
- ↑ Gehring W. 1999. Master control systems in development and evolution: the homeobox story. Yale.
- ↑ Shubin N, Tabin C and Carroll S. 1997. Fossils, genes and the evolution of animal limbs. Nature 388, 639–648.
- ↑ Shubin N, Tabin C and Carroll S. 2009. Deep homology and the origins of evolutionary novelty. Nature 457, p818–823.
- ↑ Sarich V. 1976. The panda is a bear. Nature 245, 218–220.
- ↑ Davies D.D. 1964. The giant panda: a morphological study of evolutionary mechanisms. Fieldiana Memoires (Zoology) 3, 1–339.
- ↑ Stanley Steven M. 1979. Macroevolution: pattern & process. Freeman, San Francisco. p157
- ↑ Clack, Jenny A. 2002. Gaining Ground: the origin and evolution of tetrapods. Bloomington, Indiana. ISBN 0-253-34054-3
- ↑ Home page - Jenny Clack
- ↑ Both whale evolution and early insect flight are discussed in Raff R.A. 1996. The shape of life. Chicago. These discussions provide a welcome synthesis of evo-devo and paleontology.
- ↑ 78.0 78.1 Janvier, Philip 2008. Squint of the fossil flatfish. Nature 454, 169
- ↑ Lamark J.B. 1809. Philosophie zoologique. Paris.
- ↑ Mivart St G. 1871. The genesis of species. Macmillan, London.
- ↑ Darwin, Charles 1872. The origin of species. 6th ed, Murray, London. p186–188. The whole of Chapter 7 in this edition is taken up with answering critics of natural selection.
- ↑ Goldschmidt R. Some aspects of evolution. Science 78, 539–547.
- ↑ Goldschmidt R. 1940. The material basis of evolution. Yale.
- ↑ Friedman, Matt 2008. The evolutionary origin of flatfish asymmetry. Nature 454, 209–212.
References
- Allen, Garland. Thomas Hunt Morgan: The Man and His Science, Princeton University Press, 1978 ISBN 0-691-08200-6
- Bowler, Peter J. (2003). Evolution:The History of an Idea. University of California Press. ISBN 0-52023693-9.
- Dawkins, Richard. The Blind Watchmaker, W.W. Norton and Company, Reissue Edition 1996 ISBN 0-393-31570-3
- Dobzhansky, T. Genetics and the Origin of Species, Columbia University Press, 1937 ISBN 0-231-05475-0
- Fisher, R. A. The Genetical Theory of Natural Selection, Clarendon Press, 1930 ISBN 0-19-850440-3
- Futuyma, D.J. Evolutionary Biology, Sinauer Associates, 1986, p. 12 0-87-893189-9
- Gould, Stephen Jay (2002). The Structure of Evolutionary Theory. Belknap Press of Harvard University Press. ISBN 0-674-00613-5.
- Haldane, J. B. S. The Causes of Evolution, Longman, Green and Co., 1932; Princeton University Press reprint, ISBN 0-691-02442-1
- Huxley, J. S., ed. The New Systematics, Oxford University Press, 1940 ISBN 0-403-01786-6
- Huxley, J. S. Evolution: The Modern Synthesis, Allen and Unwin, 1942 ISBN 0-02-846800-7
- Larson, Edward J. (2004). Evolution:The Remarkable History of a Scientific Theory. Modern Library. ISBN 0-679-64288-9.
- Margulis, Lynn and Dorion Sagan. "Acquiring Genomes: A Theory of the Origins of Species", Perseus Books Group, 2002 ISBN 0-465-04391-7
- Mayr, E. Systematics and the Origin of Species, Columbia University Press, 1942; Harvard University Press reprint ISBN 0-674-86250-3
- Mayr, E. and W. B. Provine, eds. The Evolutionary Synthesis: Perspectives on the Unification of Biology, Harvard University Press, 1998 ISBN 0-674-27225-0
- Simpson, G. G. Tempo and Mode in Evolution, Columbia University Press, 1944 ISBN 0-231-05847-0
- Smocovitis, V. Betty. Unifying Biology: The Evolutionary Synthesis and Evolutionary Biology, Princeton University Press, 1996 ISBN 0-691-27226-9
- Wright, S. 1931. "Evolution in Mendelian populations". Genetics 16: 97–159.
External links
- Rose MR, Oakley TH, The new biology: beyond the Modern Synthesis. Biology Direct 2007, 2:30. A review of biology in light of recent innovations since the initiation of modern synthesis.
|
||||||||||||||||||||||||||||||||